Abstract
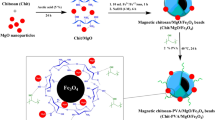
The applicability of the synthesized carboxymethyl-β-cyclodextrin-Fe3O4 nanocomposite (CM‑β-CD-Fe3O4NPs) as a novel adsorbent for eliminating Methylene blue dye (MB) from aqueous media was investigated. Various techniques including Brunauer Emmett Teller analysis (BET), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) have been used to characterize this novel adsorbent. The effect of initial concentration (C0), pH, adsorbent dosage (dose), contact time (tc), and temperature (T, K) on the removal percentage (Ad%) of MB dye onto CM-β-CD-Fe3O4NPs was studied, and the optimum value of each factor was determined (pH 6.0, dose = 0.20 g, tc = 45.0 min, and T = 298.0 K). Several isotherm models, such as Langmuir, Freundlich and Redlich–Peterson models were used to represent the adsorption experimental results, but the Freundlich model represents better the results than others. The pseudo-second-order kinetics coincided quite with the kinetic results. Based on the obtained thermodynamic values such as standard Gibbs free energy change (\(\Delta G_{{{\text{ad}}}}^{^\circ }\) < 0), standard enthalpy change (\(\Delta H_{{{\text{ad}}}}^{^\circ }\) < 0) and standard entropy change (\(\Delta S_{{{\text{ad}}}}^{^\circ }\) < 0), the nature of the adsorption process was spontaneous, exothermic and mainly physisorption.












Similar content being viewed by others
REFERENCES
V. Rocher, J. M. Siaugue, V. Cabuil, and A. Bee, J. Water Res. 42, 1290 (2008).
S. Hajati, M. Ghaedi, and H. Mazaheri, Desalin. Water Treat. 57, 3179 (2016).
R. Tang, C. Dai, C. Li, W. Liu, S. Gao, and C. Wang, J. Chem. 10, 1155 (2017).
P. Ken Gillman, Psychosomatics 51, 448 (2010).
R. Alizadeh and A. Zeidi, Adv. Environ. Res. 6, 113 (2017).
M. Ghaedi, Sh. Hajati, B. Barazesh, F. Karimi, and Gh. Ghezelbash, J. Ind. Eng. Chem. 19, 227 (2013).
S. M. Lee and S. T. Ong, APCBEE Proc. 9, 165 (2014).
D. Bahadur, D. Dutta, and D. Thakur, Chem. Eng. J. 281, 482 (2015).
K. G. Pavithra, P. S. Kumar, V. Jaikumar, and P. S. Rajan, J. Ind. Eng. Chem. 75, 1 (2019).
Y. Kuang, X. Zhang, and S. Zhou, J. Water. Res. 12, 587 (2020).
D. D. Asouhidou, Colloids Surf. A 346, 83 (2009).
C. Shan, Z. Ma, M. Tong, and J. Ni, Water Res. 69, 252 (2015).
S. Palchoudhury and J. R. Lead, Environ. Sci. Technol. 48, 14558 (2014).
A. Abdilmaleki, S. Mallekpour, and S. Borandeh, RSC Adv. 110, 1063 (2015).
F. Zhao, E. Repo, D. Yin, S. Kalliola, J. Tang, E. Yakovleva, K. C. Tam, and M. Sillanp, Sci. Rep. 10, 1038 (2017).
A. Asfaram and M. Ghaedi, RSC Adv. 6, 40502 (2016).
S. Hajati, M. Ghaedi, B. Barazesh, F. Karimi, R. Sahraei, A. Daneshfar, and A. Asghari, J. Ind. Eng. Chem. 20, 2421 (2014).
K. Kiani, S. Bagheri, and N. Karachi, and E. Alipanahpour Dil, Desalin. Water Treatm. 60, 1 (2019).
K. Boonyarattanakalin, P. Wolschann, P. Toochinda, and L. Lawtrakul, Eur. J. Pharm. Sci. 47, 752 (2012).
U. R. Bhaskara-Amrit, P. Agrawa, and M. Warmoeskerken, Autexj. 11, 94 (2011).
A. S. S. Ibrahim, A. A. Al-Salamah, A. M. El-Toni, M. A. El-Tayeb, and Y. B. Elbadawi, Biol. Electron. J. Biol. 16, 10 (2013).
H. S. Ghazimokri, M. Monajjemi, and H. Aghaie, Rev. Uni. Zulia. 29, 98 (2020).
J. Zhu, P. Wang, and M. Lu, M. J. Braz. Chem. Soc. 24, 2317 (2013).
T. K. Sen, S. Afroze, and H. M. Ang, Equilib., Water Air Poll. 218, 499 (2011).
M. Rafatullah, O. Sulaiman, R. Hashim, and A. Ahmad, J. Hazard. Mater. 177, 70 (2010).
E. Furusaki, Y. Ueno, N. Sakairi, N. Nishi, and S. Tokura, Carbohydr. Polym. 29, 29 (1996).
A. Z. M. Badruddoza, S. S. H. Goh, K. Hidajat, and M. S. Uddin, Colloids Surf., A 367, 85 (2010).
M. R. R. Kooh, M. K. Dahri, L. B. L. Lim, L. H. Lim, and O. A. Malik, Environ Earth Sci. 75, 783 (2016).
M. Auta and B. H. Hameed, J. Chem. Eng. 237, 350 (2014).
W. P. Utomo, E. Santoso, G. Yuhaneka, A. I. Triantini, M. R. Fatqi, M. F. Hudadan, and N. Nurfitria, J. Chem. 13, 104 (2019).
H. S. Ghazi Mokri, N. Modirshahla, M. A. Behnajady, and B. Vahid, Int. J. Environ. Sci. Technol. 12, 1401 (2015).
G. Mamba, X. Y. Mbianda, P. P. Govender, B. B. Mamba, and R. W. Krause, J. Appl. Sci. 10, 940 (2010).
S. Kaur and A. Gaur, Adv. Sci. 20, 1707 (2014).
B. R. Saifutdinov, V. A. Davankov, and M. M. Il’in, Russ. J. Phys. Chem. A 88, 358 (2014).
B. R. Saifutdinov, V. A. Davankov, G. A. Petukhova, M. P. Tsyurupa, Z. K. Blinnikova, and M. M. Il’in, Dokl. Phys. Chem. 462, 135 (2015).
W. Wei, L. Yang, W. H. Zhong, S. Y. Li, J. Cui, and Z. G. Wei, Dig. J. Nanomater. Bios. 10, 1343 (2015).
R. Mahini, H. Esmaeili, and R. Foroutan, Turk. J. Biochem. 24, 1(2018).
D. Pathania, S. Sharma, and P. Singh, Arab. J. Chem. 10, 1445 (2017).
S. Bagheri, H. Aghaei, M. Ghaedi, A. Asfaram, M. Monajemi, and A. A. Bazrafshan, Ultrason Sonochem. 41, 279 (2018).
S. Bagheri, H. Aghaei, M. Ghaedi, M. Monajjemi, and K. Zare, Euras. J. Anal. Chem. 13, 1 (2018).
G. H. Haghdoost, J. Phys. Theor. Chem. 15, 141 (2019).
M. Ghaedi, S. Heidarpour, S. Nasiri, S. Kokhdan, A. Daneshfar, and B. Brazesh, Powder Technol. 228, 18 (2012).
M. Toor and B. Jin, J. Chem. Eng. 187, 79 (2012).
M. Roosta, M. Ghaedi, A. Daneshfar, R. Sahraei, and A. Asghari, Ultrason. Sonochem. 21, 242 (2014).
J. Fu, Z. Chen, M. Wang, S. Liu, J. Zhang, J. Zhang, R. Han, and Q. Xu, Chem. Eng. J. 259, 53 (2015).
M. T. Yagub, T. K. Sen, and H. M. Ang, Water Air Soil Pollut. 223, 5267 (2012).
S. Bagheri, Orient. J. Chem. 32, 549 (2016).
A. A. Sabri, T. M. Albayati, and R. A. Alazawi, Korean J. Chem. Eng. 32, 1835 (2015).
ACKNOWLEDGMENTS
We would like to acknowledge Science and Research Branch of Islamic Azad University for supporting this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ghazimokri, H., Aghaie, H., Monajjemi, M. et al. Removal of Methylene Blue Dye from Aqueous Solutions Using Carboxymethyl-β-Cyclodextrin-Fe3O4 Nanocomposite: Thermodynamics and Kinetics of Adsorption Process. Russ. J. Phys. Chem. 96, 371–380 (2022). https://doi.org/10.1134/S0036024422020108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422020108